ADSORBENT TREATMENT OF COMPLEX INDUSTRIAL WASTEWATER: OPTIMIZATION OF PROCESS CONDITIONS BY RESPONSE SURFACE METHOD
Keywords:
Response surface method, Industrial wastewater, AdsorptionAbstract
The aim of this study was to use low cost adsorbent blend with synthetic zeolite in the treatment and Comparative study of the adsorbents respectively with the aim of detoxifying produced water before its safe disposal onto land or into water bodies. One week composite sampling was done every 2 hours, mixed properly and stored before the treatment processes commenced. Characterization was carried out on the produced water according to standard methods. The coconut shell and synthetic zeolite were afterward treated following standard procedures. Part of the treated coconut shell and zeolite were then mixed at ratio 1:1. The experimental parameters
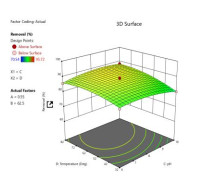
such as adsorbent dose and initial analytes concentrations, temperature and contact time were studied via design expert model. The optimum adsorption percentage occurred at adsorbent dose of 1 g, contact time at 120 minutes, pH value at 8, and temperature at 57 0C for all adsorbents were blend ratio zeolite/carbonized coconut shell was 97.33%, zeolite was 94.65% and carbonized coconut shell was 95.72%. The optimum adsorption percentage was achieved at 1 g/ 100 ml dose with blend ratio zeolite/carbonized coconut shell. High adsorption capacity and dimensionless separation parameters of the tested blend ratio zeolite/carbonized coconut shell fit
and makes it preferable, cheap and environmentally friendly alternative adsorption material.
Downloads
References
Ahmadun, F., Pendashteh, A., Abdullah L.C., Biak D.R., Madaeni S.S., Abidin Z.Z. (2009) Review of technologies for oil and gas produced water treatment. Journal of Hazardous Material, 170(2-3), 530-551pp.
Altare, C.R., Bowman, S.R., Katz, L.E., Kinney A. K., Sullivan, J.E. (2007): Regeneration and Long-term stability of surfactant modified zeolite for removal of volatile organic compounds from produced water. Microporous and Mesoporous Materials. 105(3) 305-316pp.
Emam, E.A. (2013): Modified activated carbon and bentonite used to adsorb petroleum hydrocarbons emulsified in aqueous solution. American journal of Environmental Protection.2(6), 161-169 pp.
EPA (2015):International Decontamination Research and Development Conference. U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-15/283.
Freundlich, H.M.F. (1906). Adsorption in Solutions. Journal of Physical Chemistry. 57, 384-410.
Okieimen, F.E., Okieimen C.O., Ojokoh F.I. (2005): Rubber seed shell carbon as sequestrant of heavy metals and organic compounds from aqueous solution. Indian Journal of Chemical Technology, 12, 181-186pp.[7]
AOAC (2000): AOAC official methods of analysis, 16 th ed. Association of Official Analytical Chemists,
Arlington, Virginia. 84-85pp
Downloads
Published
Issue
Section
License
Copyright (c) 2023 The Journals of the Nigerian Association of Mathematical Physics

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.